
Traditional research ethics management involves large, cumbersome forms and manual approval processes that delay project start-up, increase administrative overheads, and frustrate users.
Integrity IQ revolutionizes this process with a comprehensive SaaS platform trusted for over a decade by large, leading research intensive organisations across the globe. and complex research-intensive organizations.
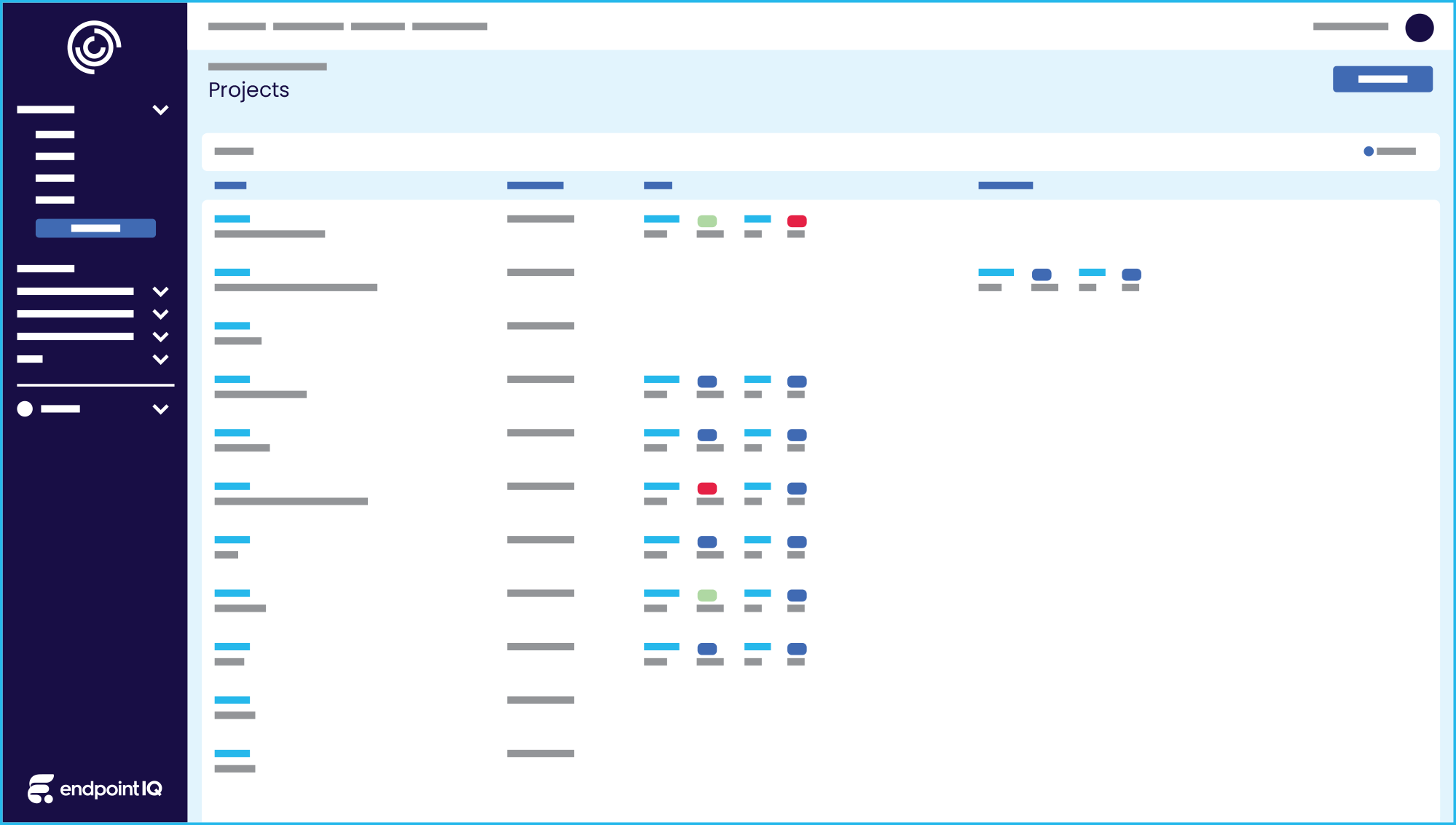
Experience the benefits of streamlined data entry, enhanced submission quality, project review tools, and improved auditability across all ethics streams. Unleash your research potential and empower your teams.
Human Ethics
Animal Ethics
Clinical Trials
Site specific assessments
Data Management Plans
OGTR (Office of the Gene Technology Regulator)
Biosafety (Haz Chems)
SOPs
Skills, Training & competencies
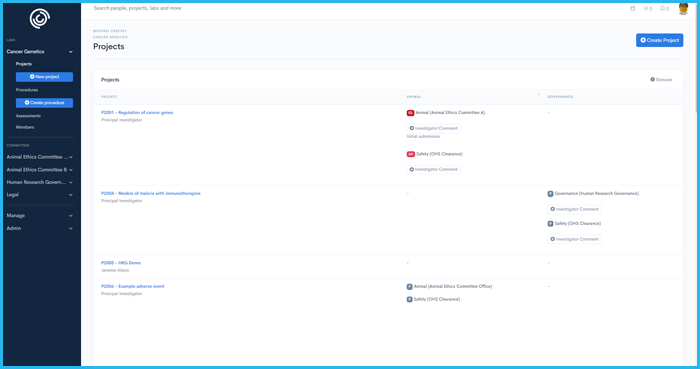
From human ethics to conflicts of interest, Integrity IQ rapidly ensures compliance across ethical principles and standards.
Strengthen oversight across diverse stakeholder groups ensuring nothing gets missed
Single source of truth for all forms & protocols, upcoming actions and reliable electronic correspondence.
Modern user experience and in-app system tools present your organisation professionally to build trust and confidence in your systems.

Endpoint IQ's systems exist to reduce administration burden and review times for research. Integrity IQ adopts an enter once, use many approach to reduce unnecessary data entry for researchers.
Make timely management decisions and ensure compliance through effective automation and notifications
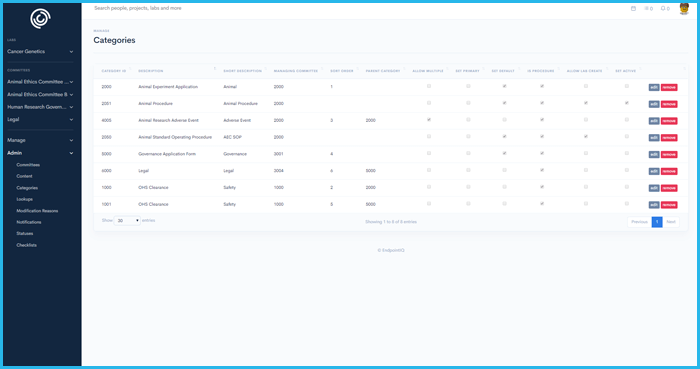
Digitally record SOP's and procedures in a single, versioned library
Obtain a project group (portfolio) approvals that are applicable to across projects (Hazardous chemicals, OGTR, risk assessments)
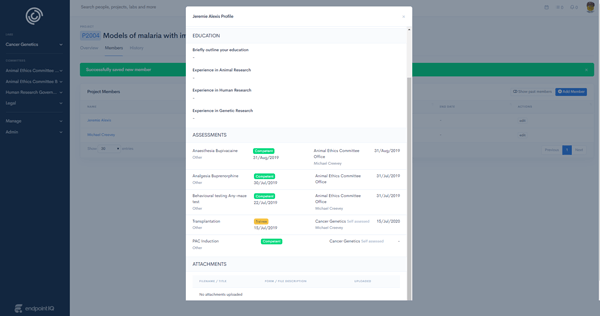
Techniques(skills) and competency assessment is stored against each member and automatically linked to applications

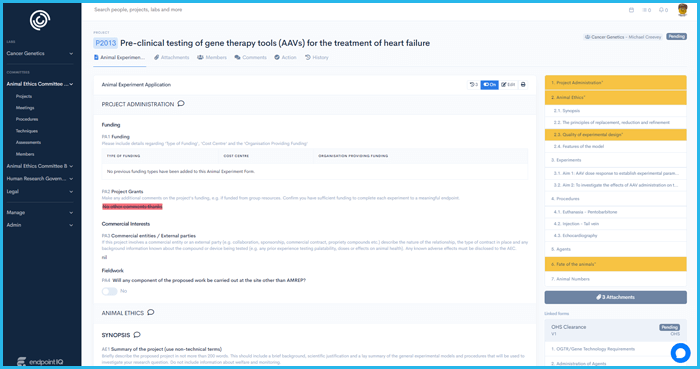
Certain approvals require project members to acknowledge approval conditions. In-app digital sign-off tools ensure complete compliance with visibility and escalation notifications over member acceptance status.
If ever there is a need for investigation, users can go back to any point in time to see what the state of a form was and what the conditions of it's approval were.
Critical notifications arising from incidents like SAE's on a clinical trial can be notified via SMS (for example) according to your policies.
Our SaaS platform transforms the traditional ethics process by replacing cumbersome forms and manual approvals with smart forms,
efficient processes, and advanced review tools. Trusted by leading, research-intensive organizations, Integrity IQ streamlines data
entry, boosts submission quality, and improves auditability.
Across all ethics & compliance streams discover how you can empower stakeholders and unlock research potential.
Seamless integration
Dynamic, API driven integrations promote efficiency and improves data integrity.
Robust and reliable
Whole-of-organisation data integrity and searchability are finally achievable.
Consistent and correct
Integrations with third party systems reduces manual data entry and opportunity for error.
Configurable notifications
Configurable in-app, email or SMS notifications and escalation pathways.
Advanced in-app form builder
Setup modern, mobile friendly and intuitively smart forms in minutes.
Users skills, training and competencies are tracked against their profile. Examples are fire training, GCP training, driver's licence or advanced animal surgery techniques. Techniques required for a project or procedure can be set by administrators and validated across approvals.

Research Grants, Commercials, HDR students
Funding Opportunities
Invoicing, Milestones, Acquittals and KPIs
Contracts and workflows
Researcher self-service and reporting

Configurable research output profiles
Research impact analysis and metrics
Automated publication harvesting
Institutional Repository
Open Access Repository